Key Takeaways:
- Compliance is a growth enabler, not just a legal requirement—top brands use it to scale faster and safer.
- Global regulations vary (FDA, EU, Health Canada), and entering new markets multiplies complexity.
- Every new SKU increases risk—from ingredient reviews to labeling and regional approvals.
- Speed-to-market without compliance gates leads to recalls, fines, and lost trust.
- Manual systems break under scale—integrated ERPs like Mar-Kov automate and futureproof compliance.
- Non-compliance costs millions in lost sales, penalties, and brand damage—automation prevents this.
When Claire’s, a beloved youth cosmetics brand, had to pull multiple products from shelves due to asbestos contamination, it wasn’t just a recall, it was a reputational crisis. Johnson & Johnson’s legal battles over talcum powder cost them billions and a massive erosion of public trust. The Honest Company learned the hard way that overstated sunscreen claims could spark regulatory scrutiny and damage consumer loyalty.
These incidents are stark reminders: in cosmetics manufacturing, compliance isn’t a sideline concern, it is the very foundation of sustainable, scalable growth. Brands that prioritize regulatory mastery expand faster, protect trust better, and outlast their competition.
At Mar-Kov, we’ve partnered with cosmetics leaders to embed compliance excellence into their operations turning regulatory hurdles into growth accelerators. Here’s how today’s top brands navigate regulatory complexity to win globally.
Understanding the Global Regulatory Environment
Scaling your cosmetics business isn’t just about increasing output it’s about navigating a maze of evolving regulations across countries and continents. From GMP standards in North America to EU labelling laws and Health Canada’s requirements, compliance isn’t optional, it’s a strategic advantage.
Before you enter a new market or onboard a new partner, you need to know what’s required, what’s at stake, and how to stay audit-ready at every turn.
Let’s break down what matters most:
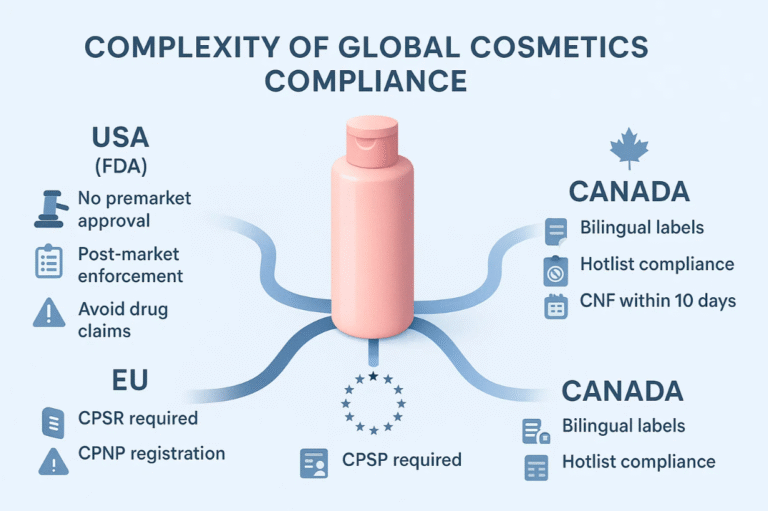
UNITED STATES
FDA Requirements
- Safety Assurance: Cosmetics must be safe for intended use. No premarket approval, but strict post-market enforcement.
- Labelling Standards: Ingredients listed by predominance; colour additives properly declared.
- Prohibited Ingredients: Banned items include mercury compounds, chloroform, and certain colour additives.
- Claims Management: Avoid drug claims unless products are registered as drugs.
EUROPE
EU Cosmetic Regulation
- Safety Reports: Mandatory Cosmetic Product Safety Reports (CPSR) by qualified professionals.
- Responsible Person (RP): A designated legal entity accountable for compliance.
- Notification: CPNP registration required before market entry.
- Banned/Restricted Ingredients: Strict lists enforced across member states.
- Labeling Requirements: Multilanguage labels, batch numbers, expiration dates.
CANADA
Health Canada Regulations
- Notification: Cosmetic Notification Form (CNF) filed within 10 days of first sale.
- Hotlist Compliance: Products must exclude prohibited substances.
- Bilingual Labeling: Labels must be in English and French.
- Marketing Claims: No therapeutic claims without additional approvals.
A surge in Canadian border seizures stems from Hotlist violations and bilingual labelling failures.
Why Regulatory Complexity Multiplies as You Scale
Success brings complexity and nowhere is that more evident than in regulatory compliance. As your cosmetic business grows, so does the web of regulations, certifications, and market specific requirements you must navigate.
Each new SKU, market entry, or faster launch timeline compounds your regulatory burden. Without the right systems in place, what once felt manageable becomes overwhelming and costly.
Here’s how scaling multiplies compliance challenges and what to watch out for before your operations outgrow your processes:
-
SKU Explosion
Launching additional product lines isn’t just a matter of increasing output each new SKU demands:
- Ingredient reformulation reviews.
- Labelling updates to meet regional compliance.
- New batch records and safety substantiation per market.
- Certification renewals where applicable (e.g., cruelty free, organic claims).
Without systematized processes, each SKU exponentially increases the compliance burden, stretching regulatory teams thin and introducing higher risks of human error.
Let’s dive deeper:
- SKU proliferation impacts not just labelling but full lifecycle documentation, from sourcing to batch release.
- Specialized SKUs (e.g., limited edition scents) often introduce unique regulatory needs, adding unexpected complexity.
- Many companies underestimate the secondary workload created by SKU variations, including unique marketing approvals, regional legal reviews, and language adaptations.
-
Market Diversification
Expanding into international markets dramatically amplifies regulatory pressure:
- Each country has unique banned substance lists.
- Claims that are acceptable in the U.S. may be illegal in the EU or Canada.
- Languages, units of measure, and regulatory filings vary.
Scaling into five new markets can multiply your compliance workload fivefold unless pre-emptive workflows and markets specific regulatory libraries are in place.
Consider this:
- EU expansion may require translation and validation in over 20 languages, each needing regulatory and legal signoff.
- Differing cosmetic definitions (e.g., antiaging creams considered drugs in some countries) necessitate reformulation or dual product strategies.
- Distributors often demand local compliance guarantees before onboarding a brand, putting extra stress on regulatory teams.
-
Speed-to-Market Pressures
In today’s competitive beauty landscape, being first can mean millions. Brands chasing aggressive launch cycles often cut corners, skipping critical compliance checks. This leads to:
- Missed label requirements.
- Unsubstantiated marketing claims.
- Product holds or recalls postlaunch.
Operational speed must be matched with embedded compliance checkpoints at every stage concept, formulation, production, and marketing.
At a more advanced level:
- Building “stage gates” into product development where compliance approvals are mandatory can save brands millions in rework and market recall costs.
- Companies that integrate compliance checks during R&D, not after, see a 40% faster time-to-market and fewer postlaunch issues.
Building a Resilient Compliance System

Fast-growing beauty brands know compliance isn’t a checklist, it’s infrastructure. As you scale, what once worked manually or across shared folders begins to crack under the weight of volume, speed, and complexity.
A resilient compliance system isn’t just about avoiding risk, it’s about enabling growth, investor confidence, and faster time-to-market. The strongest brands design compliance into their operations from day one, turning audits into non-events and regulatory shifts into opportunities.
Let’s walk through the three stages of compliance maturity and what separates high-risk operations from high-performance brands:
Stage 1: Manual Tracking (High-Risk)
- Compliance documents live in disconnected spreadsheets and email chains.
- No centralized repository for batch records or regulatory updates.
- Vulnerable to missing paperwork during audits.
In practice:
- Teams scramble during audits, often relying on personal recollections rather than verified data.
- Batch inconsistencies and gaps in labelling archives are common, risking enforcement actions.
Stage 2: Partial Automation (Risky for Scaling Brands)
- Some digital document storage (Dropbox, SharePoint), but no automated validation.
- Compliance responsibility scattered across departments.
- Heavy reliance on manual reviews, leading to bottlenecks.
The reality:
- Without real-time integration, compliance teams operate reactively, fixing errors postproduction.
- Staff turnover causes loss of compliance knowledge because systems aren’t embedded organizationally.
Stage 3: Integrated Systems (Winning Brands)
Cosmetics specific ERP systems (like Mar-Kov) automate compliance activities, including:
- Realtime batch record generation linked to production activities.
- Ingredient validation against global regulatory lists at formulation.
- Automated creation and control of multilingual, market specific labels.
- Deviation tracking and automated CAPA (Corrective and Preventive Actions) workflows.
Advanced benefits:
- Integrated platforms allow compliance teams to “design for compliance” rather than “check for compliance” post hoc.
- Centralized dashboards give C-Suite executives real-time visibility into global compliance status supporting investor confidence and faster market entries.
- Audits shift from stressful “fire drills” to routine, near effortless processes.
The True Cost of Compliance Failures

Regulatory missteps in cosmetics aren’t just paperwork problems, they’re business killers. From multimillion-dollar recalls to public brand collapses, the cost of getting it wrong can eclipse the cost of doing it right by orders of magnitude.
As you scale, your exposure multiplies. Regulatory gaps that were once survivable become existential threats. And in an age of instant social media backlash, a single oversight can cost more than just money, it can cost trust.
Here’s what’s really at stake when compliance is treated as an afterthought:
Financial Penalties and Losses
- FDA enforcement actions can lead to costly recalls, warning letters, and court settlements.
- EU regulatory failures (e.g., missing CPNP filings) can prevent market access altogether.
- Health Canada seizures or Hotlist violations can disrupt distribution networks.
After operational scaling, Niko Cosmetics discovered during an internal audit that a labelling oversight on a bestselling product could have cost them over $500,000 in recall logistics, retailer penalties, and lost seasonal promotions. The experience drove them to invest early in a robust compliance management system.
Brand Trust Erosion
- 67% of consumers say they stop purchasing from a brand after a safety issue emerges (NielsenIQ).
- Social media amplifies compliance missteps, turning minor issues into viral brand damaging events.
Operational Disruption
- Crisis management diverts executive attention from strategic growth.
- Stalled product lines create supply chain ripple effects.
- Regulatory investigations consume internal resources for months.
In short, compliance failures are existential threats to scaling beauty brands.
How Mar–Kov Empowers Cosmetics Brands to Scale Through Compliance
At Mar-Kov, we don’t view compliance as paperwork we view it as a competitive weapon. We help cosmetics brands turn regulatory complexity into a competitive edge, reducing risk, accelerating approvals, and enabling faster expansion into global markets.
With embedded compliance tools tailored to cosmetics manufacturing, we transform what was once a bottleneck into a growth engine.
Key Features:
- Batch production records automatically generated and linked to inventory.
- Ingredient validation against up-to-date global regulatory lists.
- Automated deviation tracking with CAPA workflows.
- Digital document management system ready for FDA, EU, and Health Canada audits.


CASE STUDY
Niko Cosmetics
Niko Cosmetics, a growing North American beauty brand, reduced their regulatory approval cycle time by 35% after implementing Mar–Kov ERP. They eliminated 90% of manual batch documentation errors and launched three new SKUs into the Canadian and EU markets with zero compliance delays.
Compliance Isn’t Overhead, It’s a Strategic Growth Lever.
The brands that win are those that build compliance into their operational DNA. Compliance isn’t a cost centre, it’s a brand protector, a growth enabler, and a strategic asset.
When you embed compliance early, you move faster, safer, and farther.
Mar-Kov empowers you to scale smarter, scale faster, and never fear an audit again.
Schedule a Free Mar–Kov Demo and see how smarter operations start with smarter compliance.
Frequently Asked Questions
About the Author
Alex Koves leads Mar-Kov with a focus on delivering purpose-built software tools that simplify complex manufacturing challenges. With a background in operations management, he is passionate about helping businesses optimize their processes and achieve operational excellence.

