
Is Your Cosmetics Facility Truly Audit-Ready?
With new FDA MoCRA regulations now in force, non-compliance can cost you contracts or worse. Download this free, expert-designed checklist to uncover critical gaps in your cosmetics processes before they become major issues.
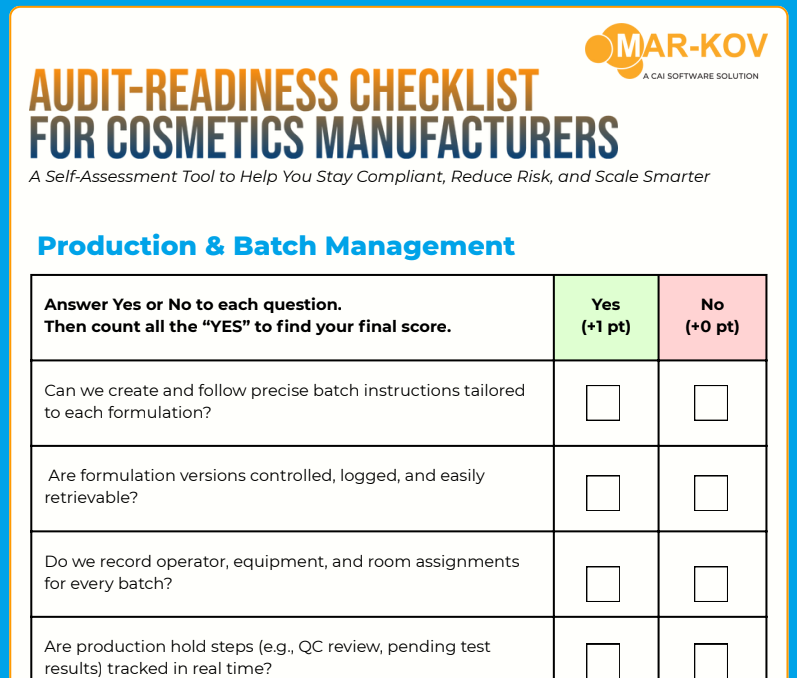
Why This Cosmetics Audit-Readiness Checklist Matters Now
A single failed audit or missing COA can trigger a costly recall, delay product release, or damage brand reputation.
FDA Requirements
The FDA now requires adherence to GMP under MoCRA
Brands Demands
Brands demand traceability, sustainability, and transparency
Better Systems
Paper-based batch records and manual QA logs are no longer enough
Market Threats
Supply chain volatility is exposing gaps in traceability and compliance
The Audit Readiness Checklist Includes
A simple, score-based GMP compliance self-assessment
Covers batch records, QA, traceability, cost control, and more
Based on ISO 22716 and FDA GMP standards
Used by production, QA, and regulatory teams at leading facilities
Immediate clarity on whether you’re Audit-Ready, Exposed, or At Risk
This checklist is built for
Production Managers
ensuring consistent execution
QA & Compliance Officers
preparing for inspections
COOs & Operations Leaders
responsible for scaling safely
Regulatory Managers
aligning with MoCRA and ISO 22716
ABOUT MAR-KOV ERP
Why Mar-Kov?
For over 30 years, Mar-Kov has helped batch manufacturers in cosmetics, personal care, and pharmaceuticals digitize operations and stay compliant. From digital batch records to traceability, ERP, and QA automation, our platform is trusted by brands and co-mans alike.
As of 2025, Mar-Kov is now part of CAI Software, giving clients even more support, innovation, and integration.
Need Help Reviewing Your Score?
We’re also offering a free 15-minute consult to review your results and identify the best next steps for improving compliance and efficiency.